Strategizing from 7 cities across the globe
A Silent Epidemic: The Hidden Triggers of Childhood Obesity
chloe batrouni,
maribelle btaich


Introduction
Childhood obesity has rapidly grown to become one of the most alarming public health crises of our time. Once considered a problem largely confined to higher socio-economic classes and affluent societies, it has now spread across the globe, affecting children from all backgrounds. Globally, the prevalence of obesity among children and adolescents aged 5–19 years has increased four-fold from 2% in 1990 to 8% in 2022 (World Health Organization, 2025). This epidemic is more than just a matter of excess weight—it is a ticking time bomb for future health complications, economic burdens, and societal challenges.
The consequences of childhood obesity extend far beyond cosmetic concerns. Obesity is strongly linked to a range of chronic conditions that may drastically alter the course of a child’s life, including type 2 diabetes, hypertension, cardiovascular diseases, and metabolic disorders. Additionally, the psychological toll of obesity— especially during a period of life as critical as childhood— is significantly profound, leaving children to grapple with struggles such as depression, low self-esteem, and social isolation, usually in addition to stigma and discrimination.
For decades, childhood obesity has often seemed to be a simple equation—too much food, not enough movement. While unhealthy eating habits, a sedentary lifestyle, and a lack of physical activity are undoubtedly significant and are not to be sidelined or ignored, they only scratch the surface of a much more complex puzzle. As hard as it may be for some to believe, beneath the surface lie hidden, frequently overlooked factors that may predispose children to obesity before lifestyle choices come into play. Emerging research is now unveiling these lesser-known influences. Genetic predispositions, early-life environmental exposures, epigenetic modifications, imbalances in gut microbiota, and even the socio-economic environment are some of the many factors that have now been shown to play pivotal roles in shaping a child's risk of obesity. These factors are not merely secondary considerations; they are foundational elements that can set the stage for the development of obesity from the earliest stages of life.
In many cases, childhood obesity is not simply a result of poor choices, but a consequence of a combination of biological, environmental, and socio-economic factors beyond a child’s control. This insight challenges the dominant narrative and calls for a shift in how we approach the issue—one that moves away from blame and shame and toward more compassionate, diverse understanding. It also urges policymakers, health professionals, and families to recognize that solving the childhood obesity epidemic requires more than just encouraging healthy habits; it requires addressing the broader, hidden factors that shape children’s health in ways we are only beginning to understand.
This research aims to explore these deeper, less visible contributors to childhood obesity, investigating the roles of genetic factors, epigenetic influence, early environmental exposures, obesogens, and food engineering that may lead to addiction, stress, psychology, and impaired gut health, while also considering socio-economic factors that influence a child’s likelihood of developing obesity. Additionally, this study will examine possible, futuristic solutions to some specific factors, ranging from simple awareness campaigns to more complex approaches such as nanotherapy, which holds promise for more targeted, individualized interventions to tackle the underlying metabolic and hormonal imbalances associated with obesity. Furthermore, the research will explore practical strategies such as public community-based health programs and policy reforms that aim to work towards healthier environments for children. In short, this study seeks to provide a more comprehensive understanding of the childhood obesity epidemic and offer insights into how prevention and intervention strategies can be improved to address its root causes.
Literature Review:
Epigenetic and Prenatal Influences:
As previously stated, obesity, particularly in children, is a complex condition influenced by various factors, including genetics, lifestyle, and environmental influences. While many of these contributors are well documented, increasing attention has been directed toward the role of epigenetic mechanisms—heritable changes in gene expression that do not alter the underlying DNA sequence—in shaping obesity risk from early development (van Dijk et al., 2015). These changes are influenced by environmental factors such as diet, stress, exposure to chemicals, and even social and psychological experiences. Epigenetic modifications—including DNA methylation, histone modification, and non-coding RNAs—can turn genes on or off without changing the genetic code itself. These processes often occur during critical windows of development, such as pregnancy and early childhood, and they can have long-lasting effects on an individual’s health outcomes.
One of the most influential periods in shaping a child’s risk of obesity occurs during pregnancy. The mother’s physical and emotional health during this time can have profound effects on the fetus’s development. Maternal stress, for example, has been shown to affect both brain development and metabolic programming in the fetus. Elevated levels of maternal cortisol, a stress hormone, can cross the placenta and impact the fetus’s hypothalamic-pituitary-adrenal (HPA) axis, potentially altering appetite regulation and metabolic function. These changes may increase susceptibility to obesity later in life. Zijlmans et al. (2015) specifically demonstrate how hormonal exposure in utero, resulting from maternal stress, can adversely affect endocrine function.
Similarly, a poor maternal diet—especially one high in fat and sugar—can result in epigenetic changes that affect the developing fetus’s energy balance regulation. Maternal high-fat diets during pregnancy are associated with rapid weight gain and increased fetal fat mass at an early stage. This dietary pattern can also activate proinflammatory cytokines, leading to maternal insulin resistance and increased free fatty acid levels in the fetus, which may predispose the offspring to obesity.
Exposure to environmental toxins during pregnancy constitutes another critical factor. Endocrine-disrupting chemicals (EDCs)—such as Bisphenol A (BPA), phthalates, and pesticides—can interfere with fetal hormone systems, contributing to long-term metabolic programming (Heindel & Blumberg, 2019).
While many studies demonstrate strong associations, it is important to note that not all findings establish direct causation. Multiple genetic, behavioral, and social factors interact in complex ways, and postnatal influences—such as early feeding practices, physical activity, and socio-economic status—also significantly affect a child’s long-term obesity risk. However, the accumulating evidence points to the critical role of maternal and environmental factors during prenatal development in shaping obesity risk, highlighting the need for targeted public health interventions and policies to address these early-life determinants (Godfrey et al., 2017).
While epigenetic modifications driven by the prenatal environment play a great role in shaping obesity risk, inherited genetic factors also significantly contribute to a child's predisposition.
Genetic Factors:
Children may be predisposed to obesity before birth if they inherit specific gene variants from their parents (Loos & Yeo, 2022). These variants interfere with our metabolism, and more precisely, with the way our bodies store and use energy, further complicating the landscape of childhood obesity. The expression of certain genes, however, can be influenced by environmental factors that affect metabolic and hormonal signals, thereby modulating gene activity without altering the DNA sequence. For instance, a child who carries a copy of the variant but exercises regularly and watches his diet might not express the gene’s effect, in contrast to a child with low levels of physical activity who eats processed food. Hence, it does not necessarily follow that children with variants will automatically become obese. It is also crucial to remember that not all children are predisposed to the same genetic risks, indicating that some children with healthy lifestyles may still become overweight, while others with similar habits remain unaffected.
One of the most important genes involved in obesity is the FTO gene, also known as the Fat Mass and Obesity-associated gene. Found on chromosome 16, this gene is highly expressed in the hypothalamus, a region in the brain that regulates our appetite, hunger, and satiety. It also regulates our energy homeostasis, or state of balance. Indeed, when the energy derived from food is greater than the energy exerted through exercise, the remaining energy will be stored as fat and thus increase the risk of obesity and weight gain. FTO variants can disrupt hypothalamic function, impairing appetite regulation. For instance, rs9939609, also referred to as “risk variant A,” is a single nucleotide polymorphism. According to the National Human Genome Research Institute, a single nucleotide polymorphism (SNP) is a genomic variant at a single base position in DNA. When present as a variant, it increases the expression of the two genes IRX3 and IRX5. These two genes play a role in energy balance and expenditure: they direct cells to become white fat cells that store energy. In the absence of their expression, these cells burn energy and instead develop into brown or beige fat cells (Claussnitzer et al., 2015). Therefore, children who carry this allele A tend to feel less full and crave more energy-dense food rich in sugar and fat. Certainly, if both copies of this allele are present, the risk for obesity will be higher, no matter the calorie intake or activity level. It is important to note that this risk develops in children between seven and twelve years of age, and not necessarily at birth. This is because hormonal changes peak at these ages and can affect the regulation of appetite. Lifestyle also has a greater influence at older ages, when children start to eat unhealthier foods, are less active, become more stressed, and sleep less. This demonstrates that environmental factors can trigger the expression of certain genes involved in obesity.
Moreover, obesity is not only determined by a single gene in our body. Rather, many genes control our appetite and fat storage, obesity being polygenic. Some of these genes include the LEP gene, which codes for the production of leptin by adipose or fat cells, and LEPR, which codes for the production of leptin receptors. If the first is mutated, leptin is not produced, and in case the second is mutated, leptin has no effect on our body. In both situations, leptin cannot effectively bind receptors in the hypothalamus and signal satiety. As a consequence, the child will remain hungry, eat more, and gain more weight. Another gene involved in the storage of fat is the Niemann-Pick disease type C1 gene, also referred to as NPC1. NPC1 encodes a protein essential for lysosomal function, which is responsible for cellular waste breakdown. For that reason, if mutated, abnormal amounts of fat and cholesterol will be stored in cells, eventually leading to weight gain and obesity. Finally, MC4R, or Melanocortin 4 Receptor, is an exception to the genes influencing obesity. It is responsible for cases of monogenic obesity, which means that this single gene is enough to cause obesity in an individual. When mutated, it dysregulates satiety and energy balance. As a matter of fact, it holds an important function in the melanocortin pathway. This pathway is stimulated by neurons activated by the hormone leptin, which signals satiety. Thus, a mutation in MC4R will result in continuous appetite and less energy expenditure (Locke et al., 2021).
The effects of these mutations highlight the value of their early detection. Unfortunately, a significant proportion of Lebanon’s population is not aware of the effects of genetic predisposition on children. People usually assume that children are “lazy” and label this as the reason they are gaining weight, while often overlooking the fact that biological factors impact obesity as well.
In addition to genetic factors, another emerging area of research is the human microbiome. As we explore the role of the microbiome, it becomes clear that its development in early life is deeply intertwined with both genetic and environmental factors.
Influence of the Gut Microbiome:
The human microbiome refers to the trillions of microorganisms, including bacteria, viruses, fungi, and other microbes, that inhabit our bodies, particularly our digestive systems. These microorganisms play a critical role in various physiological processes, including digestion, immune function, and metabolism. They help break down food, extract nutrients, and influence the way the body stores fat. Emerging evidence suggests that the microbiome plays a role in appetite regulation, fat storage, and the body’s ability to burn calories, making it a critical area of research in understanding the complex causes of childhood obesity. (Li et al., 2025). While a balanced microbiome supports healthy digestion and energy regulation, an imbalanced microbiome (dysbiosis) has been linked to metabolic dysfunction, resulting in obesity. One of the earliest and most significant influences on a child’s microbiome occurs during birth. Research has shown that babies born via cesarean section (C-section) have different microbiomes compared to those born vaginally. Vaginal birth exposes infants to beneficial bacteria from the mother’s birth canal (maternal vagina microbiota), which helps populate the infant’s gut with a healthy microbiome. In contrast, C-section babies are exposed to fewer of the “helpful” bacteria from the mother’s birth canal (Dominguez-Bello et al., 2010), which can lead to an imbalance in their gut microbiome. This early-life disruption in microbiome development has been associated with an increased risk of obesity later in life.
The maternal microbiome also plays a significant role in shaping the infant’s gut microbiome. Research has shown that the bacteria present in a mother’s gut can be passed on to the child during pregnancy, breastfeeding, and, as previously mentioned, childbirth. A healthy maternal microbiome can promote the development of a healthy gut microbiome in the infant, while an imbalanced maternal microbiome may predispose the child to obesity and other metabolic disorders. For instance, studies have found that mothers who have a high level of obesity-related bacteria in their microbiome are more likely to have obese children. The maternal microbiome is thus an essential factor in the intergenerational transmission of obesity risk.
Additionally, the use of antibiotics during infancy can further disrupt the development of the microbiome by reducing the diversity and abundance of beneficial bacteria. The extent of this disruption depends on factors such as the type, timing, and duration of antibiotic treatment. Such alterations in microbiome composition have been associated with an increased risk of obesity later in childhood. For instance, Li et al. (2022) showed that babies who take antibiotics before turning one year old will have an increased body mass index by the time they are two years and a half due to the changes in their microbiome. This disruption to the microbiome can have lasting effects, influencing not only body weight but also immune function and the risk of chronic diseases.
Emerging studies have provided further evidence of the link between microbiome imbalances and obesity. In one groundbreaking study, researchers transferred gut microbiota from obese mice to lean mice, and the latter quickly gained weight despite maintaining their diet or activity level (Ellekilde et al., 2014). This finding suggests that the gut microbiome plays a pivotal role in regulating body weight and metabolism. Subsequent human studies have corroborated these findings, showing that people with obesity tend to have a different and less diverse microbiome composition compared to individuals of healthy weight (Le Chatelier et al., 2013). Many studies also report that individuals with obesity tend to exhibit a higher ratio of Firmicutes to Bacteroidetes compared to lean individuals, although this pattern is not universally consistent (Koliada et al., 2017). This shift in microbiome composition may influence energy extraction from the diet and fat storage, potentially contributing to obesity risk.
While the microbiome plays a biological role in shaping metabolism and fat storage, another increasingly recognized contributor to childhood obesity is exposure to environmental chemicals known as obesogens.
Obesogens:
The concept of “obesogens” refers to chemicals in the environment which can disrupt hormonal pathways and alter the body’s natural mechanisms for regulating weight. They hamper the body's endocrine system, which regulates hormones such as insulin, thyroid hormone, and leptin, all of which are involved in regulating body weight. In turn, they have been found to play a significant role in the development of obesity. Obesogens do not directly cause obesity in the traditional sense of overconsumption of calories; instead, they manipulate biological processes, making the body more prone to fat accumulation and metabolic dysfunction (Amon et al., 2024).
The rise in childhood obesity coincides with an increased exposure to obesogens, particularly during critical periods of development such as prenatal exposure, infancy, and early childhood. These endocrine-disrupting chemicals (EDCs) contribute to the growing obesity epidemic, often in ways that go unnoticed until the long-term consequences manifest.
There are several key classes of chemicals that are classified as obesogens, including phthalates, bisphenol A (BPA) (both of which have been previously discussed regarding their impact during pregnancy), and organotins. These chemicals are found in a variety of everyday products, including plastic containers, food packaging, cosmetics, cleaning supplies, and pesticides. While their presence in the environment is widespread, the extent to which they contribute to childhood obesity is often underestimated. Below, we will explore some of the most prominent obesogens and how they affect the body’s metabolic processes.
Phthalates are a group of chemicals commonly used as plasticizers, added to plastics to increase flexibility. They are found in products such as plastic toys, food packaging, flooring, and personal care items like shampoo and cosmetics. Phthalates are known to be endocrine disruptors that interfere with the body’s hormonal systems. They have been shown to alter the expression of genes involved in fat metabolism and adipogenesis (the formation of fat cells). They can bind to receptors in the body that regulate fat storage, leading to an increase in fat accumulation. Animal studies have shown that this fat accumulation is especially concentrated in the abdominal region. Thus, by impairing the body’s ability to burn fat, phthalates promote long-term weight gain and obesity. Children who are exposed to phthalates during early development may be particularly vulnerable, as their bodies are still developing and more sensitive to environmental toxins.
One of the most concerning aspects of phthalate exposure is that it is often invisible. These chemicals are not always listed on product labels, and individuals may be unknowingly exposed to them on a daily basis. The ubiquity of phthalates in consumer goods means that children are frequently exposed to these chemicals through food, toys, and household products, which increases their risk of developing obesity.
Bisphenol A (BPA) is another common chemical found in plastics and food packaging. BPA is used in the production of polycarbonate plastics, which are often found in water bottles, food containers, and baby bottles. It is also used in the linings of canned goods. BPA has been classified as an endocrine disruptor because it can mimic the action of estrogen, a hormone that plays a key role in regulating metabolism and fat storage.
BPA exposure has been linked to an increased risk of obesity, particularly in children and adolescents. According to an article by Ribeiro et al. (2019), the exposure of children to BMA is linked to increased body mass index (BMI) and waist circumference. Studies have shown that BPA can interfere with the function of hormones that regulate hunger and fat storage, leading to overeating and increased fat accumulation. Furthermore, BPA has been shown to disrupt the development of the brain’s reward system, making individuals more prone to seeking out and consuming high-calorie foods. Like phthalates, BPA is pervasive in the environment, and children are at a higher risk of exposure due to the use of BPA-containing plastics in food and beverage products.
Organotins are another group of chemicals that have been identified as obesogens. These chemicals are primarily used as biocides in agriculture and are found in pesticides, fungicides, and wood preservatives. Organotins are also used in some industrial applications, including the production of PVC plastics. Research has shown that organotins can interfere with the body’s ability to regulate fat storage, leading to increased adiposity (fat accumulation). A study by Grun and Blumberg (2006) showed that organotins activate nuclear receptors that play a role in adipogenesis and the development of fat cells.
Animal studies have demonstrated that exposure to organotins can cause weight gain and increase fat mass by altering the expression of genes involved in adipogenesis and fat metabolism. In humans, exposure to organotins has been linked to an increased risk of obesity, particularly in children. Because these chemicals are used in agricultural products, children may be exposed to them through food, particularly fruits and vegetables that have been treated with pesticides.
Obesogens are a significant yet often overlooked factor in the development of childhood obesity, possibly disrupting normal growth patterns and increasing the risk of obesity later in life. As with phthalates and BPA, the risk of exposure to organotins is heightened during critical periods of development, such as pregnancy and early childhood. As the use of these chemicals continues to rise, so does the prevalence of childhood obesity, highlighting the need for greater awareness and regulation of endocrine-disrupting chemicals.
While chemical exposures like obesogens impact weight gain silently, other environmental factors, such as stress, also significantly amplify the risk of obesity by modifying appetite, sleep, hormone balance, and energy storage.
Role of Stress:
Stress, which is defined as the body’s natural response to any potential danger or challenges, affects us all on a daily basis. It is an important factor in children’s obesity, affecting them not only psychologically, as expected, but also biologically, which usually goes unnoticed. In fact, stress plays a major role in how children’s fat is metabolized in their body (Tomiyama, 2019). Long-term stress, also known as chronic stress, whether from parental pressure at home, academic pressure at school, or even significant life changes, can activate children’s hypothalamic-pituitary-adrenal axis, an endocrine system that controls the response to stress. Once activated, the brain’s coordinating center, also known as the hypothalamus, secretes corticotropin-releasing hormone (CRH). This secretion causes adrenocorticotropic hormone (ACTH) to be released from the pituitary gland, leading to the production of cortisol which increases appetite and induces cravings for high-sugar, high-calorie, and high-fat foods. Cortisol also increases the hormone ghrelin’s levels in the blood, which is particularly known for its role in causing hunger. By causing children to overeat, these factors directly contribute to obesity. Additionally, the hormone leptin, which has the opposite effect of ghrelin as it signals fullness and reduces appetite, becomes all the more resistant as chronic stress disrupts its levels. Leptin resistance often worsens as obesity develops, creating a feedback loop that complicates appetite control. Resistance to this hormone can eventually reach a point where children’s bodies no longer respond to it effectively, causing the children to remain hungry consistently.
Furthermore, when under continuous stress, children have a tendency to sleep less, as sleeping becomes more challenging. This lack of sleep directly increases ghrelin’s levels and decreases that of leptin. It also favors fat storage in our bodies. The intense cravings children subsequently feel make them more susceptible to obesity. As a matter of fact, children who suffer from sleep deprivation generally tend to have higher BMIs compared to others (Tominyama, 2019).
Hyper-palatable foods:
Another major contributor to obesity is hyper-palatable foods, which induce feelings of pleasure in the consumer. These foods include high levels of fat, sugar, carbohydrates, and sodium, and are better known under the category of fast food. The processed food industry intentionally produces these irresistible foods to maximize their profits; people will want to eat more and will purchase more of their products. Firstly, hyper-palatable foods can disrupt the effects of leptin and ghrelin, impairing the body’s natural signals for hunger and fullness. With that being said, the consumer will constantly desire eating those foods, even if they are not actually hungry.
The real question concerning the full effect of hyper-palatable foods on the brain’s reward system can be studied through the principles of neuroscience. When we eat fast food or foods high in fats, sugar, or salt, our mesolimbic dopamine system is stimulated. This system is our brain’s pleasure and motivation center. The ventral tegmental area (VTA) is part of the system, and the VTA neurons release dopamine when hyper-palatable foods are consumed. Dopamine is a neurotransmitter, or a chemical messenger, that helps us feel pleasure and joy. This heightened activation of the mesolimbic dopamine system reinforces the consumption of these foods, leading children to seek and eat them more frequently. This is concerning because it can lead to addictive behaviors. Over time, as children’s bodies become accustomed to the pleasurable effects, they require increasingly larger amounts of these foods to achieve the same level of satisfaction. This is called neuroadaptation, because the brain becomes “adapted” to the increased dopamine levels and the dopamine receptors become less sensitive to stimulation. This creates a cycle where children cannot stop consuming fast food and will thus be more prone to obesity. Furthermore, when high amounts of fat and sugar are consumed, the secretion of endorphins takes place. Endorphins are released by the pituitary gland and hypothalamus in the brain when our body feels pain or stress. Consequently, they help us feel calm, comforted, and euphoric. This creates an environment conducive to addiction, where children develop an emotional dependence on sugar and fat, making these foods increasingly irresistible to them. What further intensifies the effect of this endless cycle is another neurotransmitter or chemical messenger in our brain called serotonin, which influences our mood, satiety, and appetite. When found in low levels, this neurotransmitter makes us feel emotionally unstable and can contribute to mood disorders such as depression and anxiety, which may further affect our control over eating behavior. This is indeed the case when hyper-palatable foods are consumed: they do not provide our brain with the sufficient nutrients to produce serotonin, which leads to abnormal lower levels of the chemical. It is therefore safe to say that addiction to hyper-palatable food is strongly related to our biological process involving our brain and body. Hyper-palatable foods contribute greatly to obesity in children and adolescents (Fazzino et al., 2019).
Additionally, during puberty, the effect of these foods is enhanced because developing brains are more sensitive to reward pathways. In other words, they will further be activated by the strong dopamine levels coming from the intake of hyper-palatable foods. Teenagers will then be more likely to continue eating calorie-dense food, as they will make them feel “good.” Their brains will associate these foods with emotional comfort, encouraging addiction. And since addiction is extremely difficult to overcome, adolescents are at a greater risk of becoming obese. Moreover, their prefrontal cortex, responsible for decision-making and impulse control, is also still maturing. This goes to show that most teenagers still have not acquired a sense of self-control and they will be less likely to resist processed foods, which enhances the pattern of weight gain and obesity.
From an economic point of view, processed foods are cheaper than healthier ones. Thus, when countries are going through crises, they will have relatively higher rates of consumption of fast food than others. Citizens will consume energy-dense food with less nutrients, which puts them at a higher risk of obesity (Darmon & Drewnowski, 2008). For instance, in Beirut, many citizens struggle financially. Limited availability of affordable, healthy food options—often termed ‘food deserts’— can also exacerbate this issue, making fast food the default choice for many families. Breaking these eating patterns becomes a challenge and the promotion of addictive food intensifies their risk of obesity, type 2 diabetes, and heart diseases.
Methods:
While the literature reveals a biological set of drivers that can create predisposition, the way these dynamics manifest in real-world contexts—especially among vulnerable populations—remains underexplored. Since the expression of many of the aforementioned biological factors contributing to childhood obesity can be influenced or propagated by social environments, qualitative methods were employed to explore this interaction in depth.
Participant
A Lebanese sociologist with an interdisciplinary background in biology and sociology and experience in research on social inequality and public policy participated in the study.
Procedure
A qualitative semi-structured interview was conducted via the Webex online platform on June 5, 2025. The interview lasted approximately one hour. No audio recording was made; detailed notes were taken during the session and expanded immediately afterward to ensure accuracy.
The interview included 10 semi-structured questions designed to explore how social factors—such as chronic stress, media influence, socio-economic hardship, food marketing, and bullying—may not only affect children’s behaviors but also biologically reinforce mechanisms linked to obesity, including overeating, food addiction, stress, and hormonal imbalances. The sociologist emphasized how social pressures—including financial stress, shaming, and aggressive fast-food advertising—can activate or intensify biological responses that worsen predisposition to obesity.
Data Analysis
The notes from the interview were thematically analyzed to identify key patterns and insights regarding the interplay between social and biological factors. These findings support the argument that childhood obesity frequently arises not from individual choices alone, but from a complex combination of biological, environmental, and socio-economic influences often outside a child’s control. This study, however, has several limitations. First, the data derive from a single interview with one sociologist, which limits the diversity of perspectives and may not capture the full range of expert opinion. Second, as a qualitative study based on expert insight rather than multiple participants or quantitative data, the findings are not generalizable but rather illustrative of broader trends. Finally, the sociologist’s Lebanese context and interdisciplinary background may reflect culturally specific interpretations that may not apply universally.
Results:
The interview revealed how societal factors not only interact with but actively worsen biological predispositions to childhood obesity. Rather than mitigating inherent risks, social environments often intensify vulnerabilities through mechanisms such as chronic stress, unhealthy food exposure, and damaging cultural norms.
Body shaming was reported as particularly harmful for girls, reflecting prevailing gender norms that exacerbate psychological stress and influence unhealthy coping mechanisms. Bullying was identified as a common experience among children that contributes to emotional distress and maladaptive eating behaviors. The sociologist emphasized that these social stressors can biologically reinforce obesity through stress as an underlying factor driving emotional eating and hormonal imbalances. In this way, social perceptions of the body are not merely cultural—they have tangible physiological consequences, shaping how children relate to food, to their own bodies, and ultimately to their health: toxic diet culture, amplified by social media, perpetuates negative body image, as children are not satisfied with their appearance and therefore develop unhealthy eating habits.
Food marketing, like colorful candy aisles and advertisements linking toys and characters to unhealthy foods, was noted to intentionally target children and become an element of social pressure to incite children to fit in with their peers, imitate other kids’ unhealthy eating habits, and seek a sense of belonging. Junk food also disproportionately affects children from lower socio-economic backgrounds. This demographic is more susceptible not only due to economic limitations but also biological vulnerability to obesogens and food addiction.
Socio-economic hardship was linked to limited access to healthy foods, particularly in schools and communities with lower income levels, where healthy options are often expensive or unavailable. The sociologist discussed the concept of deviance in sociology, describing how "revenge food consumption” serves as a form of social belonging, and explained how primary and secondary socialization expose children to unhealthy dietary habits beyond parental control.
The expert stressed the necessity of involving pediatricians and other health professionals to frame childhood obesity as a scientific and medical issue rather than one of personal failure or aesthetics. Despite some existing programs in Lebanon, the sociologist noted that substantial changes remain elusive due to persistent structural challenges.
Proposed solutions included expert intervention, social media regulation to limit harmful content, and expanded school initiatives promoting healthy eating. However, the sociologist acknowledged that socio-economic and cultural factors require long-term, multifaceted approaches and cannot be quickly resolved.
Overall, these findings illustrate that society often compounds biological predispositions to obesity rather than alleviating them. The complex interplay of social pressures, economic barriers, and cultural norms creates an environment that worsens biological risks, emphasizing the need for holistic interventions addressing both social context and biological vulnerabilities.
Discussion:
Discussions about childhood obesity often remain fragmented and incomplete, focusing either on biological factors or social behaviors linked to it. Our findings have bridged that gap by showing that the more subtle drivers of obesity have an intricate interplay with biosocial factors and environment. Indeed, not all predisposition is solely biological, especially in the modern Lebanese context. The findings reveal that childhood obesity is far from a simple issue of individual choice or isolated biology, it indeed emerges from a dynamic system in which social stressors such as chronic financial hardship, bullying, and body shaming interact with biological vulnerabilities to create a reinforcing cycle. Likewise, the aforementioned biological vulnerabilities that can be modified by the environment can worsen existing non-modifiable ones (like genetics). In other words, people who are genetically predisposed are often targeted by social factors that intensify the risk of obesity.
In the interview, it became clear how stress-related hormonal changes can trigger emotional eating and food addiction, behaviors often perpetuated by aggressive marketing tactics targeting children, particularly those from lower socio-economic backgrounds. This vulnerable group is disproportionately affected by limited access to healthy foods and heightened exposure to obesogenic environments. Moreover, chronic stress from socio-economic hardship or bullying does not merely co-exist with obesity, it actively shapes physiological pathways— like hormonal and neurochemical changes mentioned above that exacerbate obesity risks. This aligns with epigenetic research demonstrating how environmental stressors can alter gene expression related to metabolism and fat storage in the child’s future offspring.


Download the full document
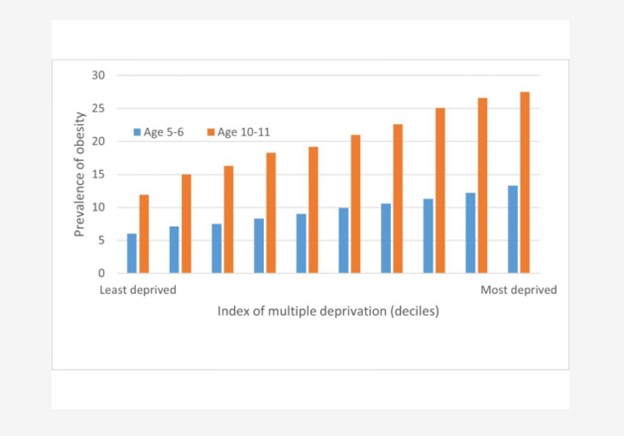
Figure 1: Child Obesity Prevalence at Ages 5–6 and 10–11 According to a Composite Neighbourhood Deprivation Measure
The bar chart above clearly demonstrates a strong, positive correlation between socio-economic deprivation and childhood obesity prevalence in England. As the Index of Multiple Deprivation (IMD) increases (shifts from the least to the most disadvantaged families) the rates of obesity rise significantly for both age groups (5–6 and 10–11 years). In the most deprived areas, obesity prevalence in the older group exceeds 25%, while it remains under 10% in the least deprived. Importantly, these patterns reflect a broader, universal relationship between socio-economic status and health outcomes that is not unique to England. Similar mechanisms of deprivation impacting childhood obesity are expected to operate in many other different countries, where socio-economic inequalities and neighborhood-level deprivation also affect health risks from a young age.
Moreover, the research underlines the significance of cultural norms and toxic diet culture, especially in perpetuating negative body image and psychological stress among children, which further fuels unhealthy coping mechanisms as well as the stress and food addiction pattern. Junk food, with marketing strategies and secondary socialization effects, often becomes an element of peer pressure and of fitting in. However, its end result of obesity turns into an element of bullying. The sociologist’s insights stressed the necessity of framing childhood obesity not as a moral failing but as a medical and social issue shaped by forces often beyond an individual child’s control. Indeed, the findings of this study aligns with and extends the understanding of childhood obesity as a biopsychosocial model: while genetic and hormonal factors contribute to obesity risk, they are often activated, highlighted or intensified by adverse social and consequently psychological conditions, creating a feedback loop that perpetuates obesity from childhood and onwards. Stressors (which, as mentioned, are a subtle obesity driver) disproportionately affect children from lower socio-economic groups, creating health disparities. Food marketing, on another hand, reinforces and encourages unhealthy behaviors, especially among vulnerable children. This vulnerability might stem from being already targeted by social factors concerning their weight (and therefore possibly already biologically or socially predisposed), hence the loop that society does not help in ending.
Children’s eating habits are also mainly embedded within cultural and social norms, not merely individual or familial choices (e.g. the concept of emotional or revenge eating and deviance from the healthy food they are being judged into choosing) reflecting the pervasive impact of diet culture, social media, body image and even socio-economic background on the destiny of one’s health since their first steps. To better illustrate this feedback loop, one could consider the example of a child who is genetically predisposed to retain more weight due to her metabolism and family history. Although her weight falls within a medically healthy range, societal standards and subtle cues from adults around her make her feel “too big.” This internalized body dissatisfaction leads to chronic stress, which in turn disrupts her hormonal balance, slowing her metabolism further and increasing fat storage. The dissatisfaction from that weight gain itself drives emotional eating, especially of hyper-palatable foods, reinforcing patterns of food addiction as a form of emotional coping. She becomes trapped in a cycle where her biology is aggravated by social pressure, which then feeds back into behavior and biology, worsening the very issue that caused her distress in the first place. Reframing obesity as a medical scientific issue rather than a reason to sink into psychological stress and body dissatisfaction that may worsen the obese child’s condition is an urgent matter to be addressed.
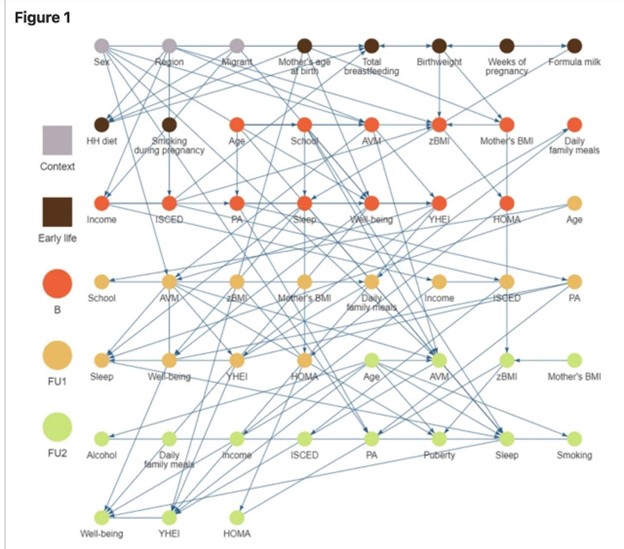
Figure 2: Systems-Based Risk Map for Obesity in European Children
Figure 2 presents a complex network map illustrating the multifactorial determinants of childhood obesity across various developmental life stages, contextual factors, and health outcomes. The causal graph highlights interactions among variables such as socio-economic context (e.g., income, education), early life factors (e.g., maternal BMI, birthweight, formula feeding), and behavioral patterns (e.g., sleep, physical activity, diet). Nodes are color-coded by life stage or data collection point (e.g., baseline, follow-up 1, follow-up 2), and the dense web of connections underscores the cumulative and interacting nature of obesity risk factors. This model emphasizes that childhood obesity cannot be explained by a single cause; rather, it is shaped by intersecting influences over time, where biology is shown to meet socio-economics and psychology once again.
The emphasis on social determinants highlights that true, meaningful change requires tackling systemic inequities and norms that drive obesity risk— such as access to affordable healthy food, recreational content for kids that is safe from toxic and addictive material, addressing bullying and media regulation (regarding body image, self worth, and eating habits encouragement) as well as psychological well-being. This research powerfully illustrates the critical necessity of shattering the often-prevailing reductionist narratives that frame obesity as simply a matter of “eat less, move more”. In doing so, we move closer to a future where childhood obesity is not a chronic, intractable burden but a preventable condition rooted in a fairer, healthier society. Schools, being central socialization agents where food environments shape long-term habits, need effective policies to reduce the toll of unhealthy behaviors on children’s lives. National nutrition standards which call for the replacement of hyper-palatable and obesogen filled foods in canteens and cafeterias with fulfilling and nutritionally valuable foods would be an important first step. Moreover, informed physical education programs that emphasize well-being and inclusivity rather than competition should be put in place. Likewise, to reduce the emotional and biological impact of aggressive advertising on vulnerable children, policymakers should limit the targeting of younger people by restricting junk food advertising during children’s programming or on platforms with high child viewership. Society would also benefit from prohibiting the use of cartoon mascots, toys, or games as a part of food marketing strategies aimed at children. These strategies aim not only to reduce unhealthy exposure but to disrupt the manipulation of biological cravings by corporate interests exploiting children’s neurodevelopmental susceptibility to reward cues. In addition to that, mitigating the emotional and biological toll of bullying, body shaming and chronic stress or lack of sleep is crucial to solving the issue of rising obesity tolls. Policies should therefore expand school-based mental health services, particularly in underserved communities, and integrate neutral and objective nutritional counseling and mental health screening into regular pediatric check-ups. Pediatricians as well as school nurses should be trained to approach obese children in a weight-neutral, trauma-informed manner that acknowledges the interplay between biology, stress, and social circumstances without shaming the child or making them feel “lesser-than”— especially since current medical models might reinforce stigma rather than address the roots of obesity. Parents would also benefit from normalizing conversations around emotional eating, reducing shame, and increasing awareness of obesity as an issue that can be solved— predisposition is not a condemnation, and remains subject to solutions as long as it is tackled with care and empathy.
At the structural level, obesity cannot be disentangled from poverty, inequality and environmental injustice. Children in food-insecure households are more likely to consume calorie-dense, nutrient-poor foods and experience chronic stress or lack of sleep. Therefore, governments must prioritize anti-poverty programs that provide families with stable access to affordable groceries and ensure safe outdoor play areas, green spaces, and walkable neighborhoods in historically neglected communities.
In the long run, however, novel effective solutions may arise and modify obesity and its less modifiable predisposing factors as we see them today: cutting-edge biomedical approaches such as nanotechnology-based therapies and epigenetic gene editing may offer new hope in addressing biological vulnerabilities to obesity—especially those previously considered irreversible (Long et al., 2024). While this paper emphasizes how social and environmental pressures intensify childhood obesity, future technologies may directly intervene at the biological level to reduce risk from within the body. For example, nanotechnology involves designing extremely small particles—measured in billionths of a meter—that can enter specific tissues or cells and carry out targeted actions. One promising area is the stimulation of “fat browning,” which refers to converting unhealthy white fat (that stores energy) into brown fat (that burns energy). Certain metal nanoparticles can produce heat when activated by light or external energy, causing fat cells to behave more like calorie-burning brown fat—effectively raising a child’s metabolism (Jiang et al., 2017). Other nanoparticles are being developed to reduce inflammation, a biological stress response that worsens obesity, by delivering anti-inflammatory drugs directly to immune cells involved in that inflammation.
Additionally, epigenetic therapies—which target the way genes are expressed without altering the DNA code itself—are being studied for their potential to "switch off" genes linked to obesity, such as those affecting appetite or fat storage (Chen et al., 2024). Gene therapy using viral vectors (harmless viruses designed to carry therapeutic material) could, in the future, reprogram how a child’s body handles sugar or fat, making them more resilient to the effects of poor diet or chronic stress. Some researchers are also exploring how editing the gut microbiome—the community of bacteria in the digestive system—could help restore healthy metabolism and reduce cravings since the microbiome affects both digestion and mood.
Though these therapies are still in early stages, futuristic, and raise ethical and accessibility questions, they represent a transformative shift: from managing obesity with surface-level tools to redefining obesity itself as biologically modifiable. If paired with strong social and policy reforms, these biomedical advances could help reshape the future—transforming what once seemed an inescapable predisposition to something that can be prevented, treated, or even reversed.
Finally, ethical responsibility is central when it comes to obesity in children. Blaming children for their weight is not only misguided but deeply unethical, especially considering the complex web contributing to their health positively or negatively, and that lies out of their control. Yet in today’s society, children who are obese are strongly subjected to stigma and social exclusion. For this to stop causing psychological harm and for meaningful change to happen, media creators, parents, teachers, and healthcare professionals must consciously avoid derogatory comments regarding weight— whether directed at the child or others— in the presence of children. They are ethically responsible not to showcase their bias to children and normalize harmful self-perceptions, as the latter are strongly receptive to observational learning. Ethically, adults have a duty to create environments where all children—regardless of body size—feel safe, accepted, and supported. Reducing obesity stigma is not a matter of correctness or politeness but a moral obligation: when society stops positioning weight as a measure of worth, children can begin to view health as something they deserve, not something they must attain to satisfy societal judgement. It is not the child’s plate that is broken, but the system surrounding them— feeding stress, shame, addiction and scarcity into their biology.
Future studies regarding obesity in children must also uphold the highest ethical standards. They should include consent from both guardians and their children in order to limit the damage to the children during the collection of data as much as possible. Research design must prioritize the well-being of child participants and avoid reinforcing shame. Ultimately, if combating childhood obesity is truly the goal of the scientific community, it requires compassionate, ethical engagement at every level of society— from research labs to school hallways.
Conclusion
This research has shown that childhood obesity is far more than a simplistic equation of diet and exercise; it may often include a profound and intricate challenge rooted in a complex interplay of biological vulnerabilities and powerful societal forces, many of which operate beyond a child’s immediate control. Our investigation, particularly through qualitative insights, reveals how these factors form a reinforcing biosocial loop, amplifying risks and challenging conventional narratives. Specifically, insights from this study illuminated how widespread societal pressures—ranging from chronic stress and economic insecurity to pervasive food marketing and toxic cultural norms—do not merely encourage unhealthy behaviors, but actively interact with and exacerbate genetic predispositions, epigenetic modifications, gut microbiome imbalances, and the physiological responses to obesogens, thereby biologically amplifying obesity risks. It has been affirmed that these external forces can trigger and intensify underlying biological vulnerabilities, creating a dynamic and often inescapable cycle. Addressing childhood obesity therefore transcends the notion of individual willpower; it emphatically demands holistic, compassionate, and multidisciplinary interventions that fully account for the complex interplay between a child's biological makeup and their broader social and environmental context. This necessitates a fundamental shift away from blame and towards systemic solutions that uphold the inherent dignity and health of every child. While the long-term horizons of emerging technologies like nanotechnology and epigenetic therapies indeed offer an exciting promise for addressing less modifiable predispositions, our immediate efforts must concentrate on actionable, policy-driven interventions. This includes reducing the pervasive stigma associated with weight, improving equitable access to healthy, supportive environments, and implementing robust supportive policies, such as stricter regulations on food marketing to children, expanded school nutrition programs, and comprehensive mental health services. Ultimately, fostering healthier generations and truly rewriting the narrative around childhood obesity hinges on our collective courage to confront all its root causes—both the evident and the 'unseen' forces. This requires not just empathy and innovation, but a sustained, unwavering commitment from individuals, communities, and policymakers to dismantle systemic barriers and build a world where every child has the equitable opportunity to thrive, free from preventable health burdens and societal judgment.
References
Amon, M., Kek, T., & Klun, I. V. (2024). Endocrine disrupting chemicals and obesity prevention: Scoping review. Journal of Health, Population and Nutrition, 43, Article 138. https://doi.org/10.1186/s41043-024-00627-y
Chen, P., Wang, Y., Chen, F., & Zhou, B. (2024). Epigenetics in obesity: Mechanisms and advances in therapies based on natural products. Pharmacology Research & Perspectives, 12(1), e1171. https://doi.org/10.1002/prp2.1171
Claussnitzer, M., Dankel, S. N., Kim, K. H., Quon, G., Meuleman, W., Haugen, C.,.. & Kellis, M. (2015). FTO obesity variant circuitry and adipocyte browning in humans. The New England Journal of Medicine, 373(10), 895–907. https://doi.org/10.1056/NEJMoa1502214
Darmon, N., & Drewnowski, A. (2008). Does social class predict diet quality? The American Journal of Clinical Nutrition, 87(5), 1107–1117. https://doi.org/10.1093/ajcn/87.5.1107
Dominguez-Bello, M. G., Costello, E. K., Contreras, M., Magris, M., Hidalgo, G., Fierer, N., & Knight, R. (2010). Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proceedings of the National Academy of Sciences, 107(26), 11971–11975. https://doi.org/10.1073/pnas.1002601107
Ellekilde, M., Selfjord, E., Larsen, C. S., Jakesevic, M., Rune, I., Tranberg, B., ... & Hansen, A. K. (2014). Transfer of gut microbiota from lean and obese mice to antibiotic-treated mice. Scientific Reports, 4, 5922. https://doi.org/10.1038/srep05922
Fazzino, T. L., Rohde, K., & Sullivan, D. K. (2019). Hyper-palatable foods: Development of a quantitative definition and application to the US food system database. Obesity, 27(11), 1761–1768. https://doi.org/10.1002/oby.22639
Foraita, R., Witte, J., Börnhorst, C., Gwozdz, W., Pala, V., Lissner, L., Lauria, F., Reisch, L. A., Molnár, D., De Henauw, S., Moreno, L., Veidebaum, T., Tornaritis, M., Pigeot, I., & Didelez, V. (2024). A longitudinal causal graph analysis investigating modifiable risk factors and obesity in a European cohort of children and adolescents. Scientific Reports, 14(1). https://doi.org/10.1038/s41598-024-56721-y
Godfrey, K. M., Reynolds, R. M., Prescott, S. L., Nyirenda, M., Jaddoe, V. W. V., Eriksson, J. G., & Broekman, B. F. P. (2017). Influence of maternal obesity on the long-term health of offspring. The Lancet Diabetes & Endocrinology, 5(1), 53–64. https://doi.org/10.1016/S2213-8587(16)30107-3
Grün, F., & Blumberg, B. (2006). Environmental obesogens: Organotins and endocrine disruption via nuclear receptor signaling. Endocrinology, 147(6 Suppl), S50–S55. https://doi.org/10.1210/en.2005-1129
Heindel, J. J., & Blumberg, B. (2019). Environmental obesogens: mechanisms and controversies. Annual Review of Pharmacology and Toxicology, 59, 89–106. https://doi.org/10.1146/annurev-pharmtox-010818-021304
Jiang, C., Ting, A. T., Tian, Y., Chen, Y., & Wu, X. (2017). Dibenzazepine-loaded nanoparticles induce local browning of white adipose tissue to counteract obesity. Molecular Therapy, 25(7), 1718–1729. https://doi.org/10.1016/j.ymthe.2017.05.020
Koliada, A., Syzenko, G., Moseiko, V., Budovska, L., Puchkov, K., Perederiy, V., Gavalko, Y., Dorofeyev, A., Romanenko, M., Tkach, S., Sineok, L., Lushchak, O., & Vaiserman, A. (2017). Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiology, 17(1), 120. https://doi.org/10.1186/s12866-017-1027-1
Le Chatelier, E., Nielsen, T., Qin, J., Prifti, E., Hildebrand, F., Falony, G., ... & Ehrlich, S. D. (2013). Richness of human gut microbiome correlates with metabolic markers. Nature, 500(7464), 541–546. https://doi.org/10.1038/nature12506
Li, P., Chang, X., Chen, X., Wang, C., Shang, Y., Zheng, D., ... & Qi, K. (2022). Early-life antibiotic exposure increases the risk of childhood overweight and obesity in relation to dysbiosis of gut microbiota: A birth cohort study. Annals of Clinical Microbiology and Antimicrobials, 21(1), 46. https://doi.org/10.1186/s12941-022-00535-1
Li, S., Ma, X., Mei, H., Chang, X., He, P., Sun, L., Xiao, H., Wang, S., & Li, R. (2025). Association between gut microbiota and short-chain fatty acids in children with obesity. Scientific Reports, 15, Article 483. https://doi.org/10.1038/s41598-024-84207-4
Locke, A. E., Kahali, B., Berndt, S. I., Justice, A. E., Pers, T. H., Day, F. R., … & Speliotes, E. K. (2021). The genetics of obesity: from discovery to biology. Nature Reviews Genetics. https://doi.org/10.1038/s41576-021-00414-z
Long, Y., Zhang, X., Chen, H., & Liu, J. (2024). Epigenetic modifications in obesity-associated diseases. MedComm, 5(1), e496. https://doi.org/10.1002/mco2.496
Loos, R. J. F., & Yeo, G. S. H. (2022). The genetics of obesity: From discovery to biology. Nature Reviews Genetics, 23(2), 120–133. https://doi.org/10.1038/s41576-021-00414-z
Ribeiro, C., Mendes, V. G., Peleteiro, B., Delgado, I., Araújo, J., Aggerbeck, M., Annesi-Maesano, I., Sarigiannis, D. A., & Ramos, E. (2019). Association between the exposure to phthalates and adiposity: A meta-analysis in children and adults. Environmental Research, 179, 108780. https://doi.org/10.1016/j.envres.2019.108780
Social disparities in child obesity: a report from the STOP project | World Obesity Federation. (n.d.). World Obesity Federation. https://www.worldobesity.org/news/social-disparities-in-child-obesity-a-report-from-the-stop-project
Tomiyama, A. J. (2019). Stress and obesity. Annual Review of Psychology, 70, 703–718. https://doi.org/10.1146/annurev-psych-010418-102936
Van Dijk, S. J., Molloy, P. L., Varinli, H., Morrison, J. L., Muhlhausler, B. S., & Members of EpiSCOPE. (2015). Epigenetics and human obesity. International Journal of Obesity, 39(1), 85–97. https://doi.org/10.1038/ijo.2014.34
World Health Organization. (2025). Prevalence of obesity among children and adolescents aged 5 to 19 years. Retrieved June 4, 2025, from https://data.who.int/indicators/i/C6262EC/EF93DDB
Zijlmans, M. A. C., Riksen-Walraven, J. M., & de Weerth, C. (2015). Associations between maternal prenatal cortisol concentrations and child outcomes: A systematic review. Neuroscience & Biobehavioral Reviews, 53, 1–24. https://doi.org/10.1016/j.neubiorev.2015.02.015
